
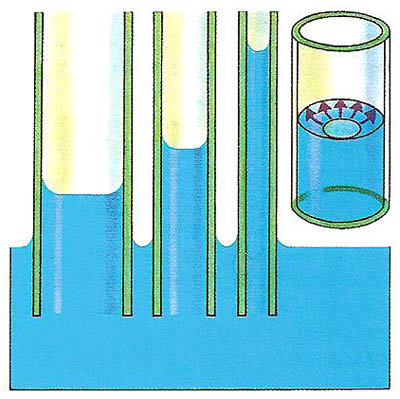
Using this formula, note the effect of the radius of the tube.

Capillary Action UsesĬapillary action has several uses. The movement of water up the stems and trunks of plants (transpiration) involves capillary action, but it also relies on evaporation from leaves and osmotic pressure from roots. Fabrics that wick away perspiration also use capillary action.Paper towels wick up water using capillary action.Paint brushes and lamp wicks pick up liquid the same way. A candle wick absorbs liquid wax that constantly supplies a candle flame.The lacrimal ducts (tear ducts) of the eyes continuously drain tears from the eye surface.Capillary action causes the rise of damp in concrete and drywall.When you place a straw in a glass of water, the liquid level within the straw is higher than the height of the water in the glass.There are many familiar examples of capillary action in everyday life: For example, the meniscus of water in plastic is nearly flat. The meniscus shape depends on both the composition of the liquid and that of the container. For example, water forms a concave meniscus in glass. A concave meniscus forms when molecules are more attracted to the container than they are to each other. For example, mercury forms a convex meniscus in glass. However, liquids like mercury rise to a level that is lower than that of the liquid surrounding the tube.Ī convex meniscus forms when molecules are more attracted to each other than they are to the container. Many liquids act like water and rise in a capillary tube. Gravity affects how far a liquid rises within a capillary, as it exerts a downward pull on the liquid in a vertical tube.However, water curves toward the container wall, while mercury forms a rounded shape in the center of a capillary.

In a narrow tube, the meniscus formed by these two liquids is curved. Water and mercury both have high surface tension. Surface tension is the tension on a liquid at its interface with air that minimizes the liquid surface area.In contrast, mercury atoms stick well to each other, but are not as attracted to a container surface. Water molecules stick to glass and plastic surfaces. Adhesion describes how well liquid molecules stick to surfaces.In the case of water molecules, cohesion is high because of hydrogen bonding between the molecules. Cohesion occurs when liquid molecules stick to each other.The three forces largely responsible for capillary action are surface tension, cohesion, and adhesion. Forces in Capillary Action – How It Works In fact, liquids often rise in narrow tube in opposition to gravity. Capillary action does not require the force of gravity.

Other names for the phenomenon are capillarity, capillary motion, and wicking. For example, if you place a thin tube into water, the water flows up the the tube. This entry was posted on Februby Anne Helmenstine (updated on March 30, 2022)Ĭapillary action is fluid flow through a narrow tube or space from surface tension, cohesion, and adhesion.Ĭapillary action is fluid flow through a narrow tube or space from surface tension, cohesion, and adhesion.


 0 kommentar(er)
0 kommentar(er)
